Upadacitinib API in Ulcerative Colitis Treatment
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) that significantly impacts patients’ quality of life. The search for effective treatments is ongoing, and recent advances have brought Upadacitinib, a Janus kinase (JAK) inhibitor, into the spotlight. This article delves into the efficacy and applications of Upadacitinib API (Active Pharmaceutical Ingredient) in treating ulcerative colitis, along with its pricing and manufacturing details.
What is Upadacitinib API
Upadacitinib API, or active pharmaceutical ingredient, represents the core therapeutic component of Upadacitinib, a potent and selective Janus kinase (JAK) inhibitor. This compound plays a crucial role in managing various inflammatory conditions by targeting specific signaling pathways within the immune system.
Mechanism of Action
Upadacitinib exerts its therapeutic effects primarily by inhibiting Janus kinases, particularly JAK1. These enzymes are essential in transmitting signals involved in immune responses and inflammation. By blocking JAK1, Upadacitinib disrupts the signaling cascade that leads to the production of inflammatory cytokines. This modulation helps to reduce inflammation and regulate immune responses, thereby alleviating symptoms associated with inflammatory diseases like rheumatoid arthritis, atopic dermatitis, psoriatic arthritis, and ulcerative colitis (UC).
Medical Uses
Initially approved for the treatment of rheumatoid arthritis, Upadacitinib has demonstrated efficacy and safety profiles that have led to its approval for other inflammatory conditions. These include atopic dermatitis and psoriatic arthritis, expanding its therapeutic applications beyond its initial indication. Particularly notable is its effectiveness in managing symptoms of ulcerative colitis, a type of inflammatory bowel disease (IBD). Upadacitinib’s ability to specifically target and modulate immune responses in the gut has marked it as a significant advancement in the treatment of UC, providing new options for patients who do not respond well to conventional therapies.
In summary, Upadacitinib API represents a cutting-edge therapeutic approach in the field of immunology and inflammatory disease management. By selectively inhibiting JAK1, it offers a targeted mechanism to alleviate symptoms and improve quality of life for patients suffering from various chronic inflammatory conditions.
Upadacitinib for Ulcerative Colitis
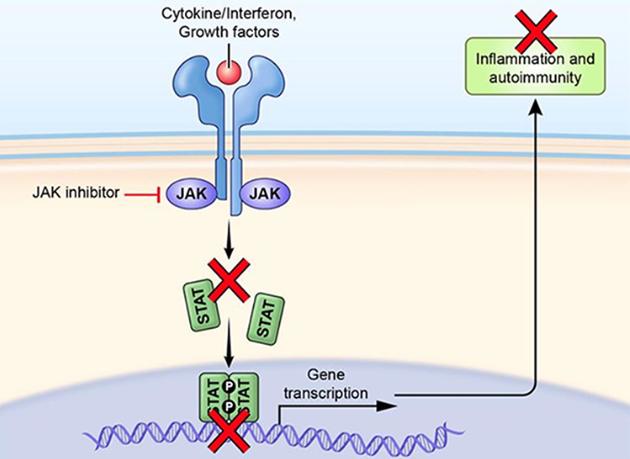
Upadacitinib (UPA) is emerging as a promising therapeutic option for patients with moderate to severe ulcerative colitis (UC) who haven’t responded adequately to conventional treatments like corticosteroids or aminosalicylates. Here’s a deeper dive into UPA’s potential in UC management:
1. Clinical Trial Results
Several clinical trials have evaluated UPA’s efficacy and safety in UC patients. These studies generally included patients with moderately to severely active UC who had an inadequate response to conventional therapies. The results have been encouraging:
- Induction of Remission: Studies demonstrated that UPA effectively induced clinical remission in UC patients. Remission is characterized by a significant reduction or absence of symptoms like rectal bleeding, stool urgency, and abdominal pain.
- Maintenance of Remission: UPA has shown promising results in maintaining remission in patients who initially respond well to treatment. This is crucial for long-term disease management and improved quality of life.
- Improvement in Symptoms: UPA treatment is associated with significant improvements in UC symptoms, including a reduction in stool frequency and urgency, rectal bleeding, and abdominal pain. This translates to a notable improvement in patients’ daily lives.
- Mucosal Healing: Upadacitinib has also shown the potential to promote mucosal healing in the colon. Mucosal healing refers to the restoration of a healthy lining in the colon, which is critical for preventing further inflammation and complications.
2. Advantages of Upadacitinib:
- Oral Administration: UPA is available in tablet form, offering a convenient and non-invasive treatment option compared to injectable medications.
- Targeted Therapy: UPA acts specifically on the immune system, potentially leading to fewer side effects compared to broad-spectrum medications like corticosteroids.
- Rapid Relief: Studies suggest that UPA can induce remission and improve symptoms relatively quickly, offering faster relief for patients.
Upadacitinib is currently undergoing further clinical trials to confirm its long-term efficacy and safety in UC treatment. Additionally, researchers are exploring its potential role in combination therapies with other medications for even better UC management.
Upadacitinib Price
The cost considerations surrounding Upadacitinib are crucial for patients, healthcare providers, and healthcare systems alike. As a newer therapeutic option with targeted efficacy in inflammatory conditions like ulcerative colitis (UC), understanding its pricing dynamics is essential for informed decision-making.
Factors Influencing Upadacitinib Price
Several factors contribute to the pricing of Upadacitinib. The cost of raw materials, the complexity of its manufacturing process, and research and development expenses all influence its final price. Additionally, regulatory approval costs and the need for ongoing pharmacovigilance add to the overall expense of bringing Upadacitinib to market. The exclusivity provided by patents also impacts pricing, as manufacturers seek to recoup investments made in developing and testing the medication.
Upadacitinib Price Comparison
Comparing Upadacitinib’s cost with other treatments for UC reveals its competitive position within the market. While initially higher in price due to its innovative nature and targeted mechanism of action, Upadacitinib’s potential to induce and maintain remission may justify its cost effectiveness over time. Cost-effectiveness analyses often consider factors such as reduced hospitalizations, surgeries, and improved quality of life associated with effective disease management.
Insurance coverage and patient assistance programs also play significant roles in mitigating out-of-pocket costs for patients. Pharmaceutical companies frequently offer financial assistance programs to eligible patients, ensuring access to Upadacitinib without financial hardship.
The availability and accessibility of Upadacitinib can vary by region and healthcare system. Negotiations between pharmaceutical companies and healthcare payers influence pricing agreements, affecting its affordability and availability in different markets. Generic competition, when introduced, may eventually lower prices, enhancing access for a broader patient population.
Qingmu: Upadacitinib Manufacturer in China

Qingmu Pharmaceutical (Qingmu) is a reputable Chinese manufacturer with a growing presence in the global market for Active Pharmaceutical Ingredients (APIs). They offer Upadacitinib API, a key component in medications for treating inflammatory conditions like UC. Here’s why Qingmu might be a strong partner for sourcing Upadacitinib API:
- Experience and Expertise: Qingmu boasts extensive experience in developing and manufacturing high-quality APIs. Their website highlights their cGMP (Current Good Manufacturing Practice) compliance and advanced quality control systems, ensuring consistent and reliable Upadacitinib API production.
- Focus on Innovation: Qingmu prioritizes innovation and technological advancements in their manufacturing processes. This commitment could translate to cost-effective Upadacitinib API production, potentially benefiting both pharmaceutical companies and ultimately, patients.
- Global Reach: Qingmu caters to an international market, possessing the infrastructure and expertise for efficient global distribution of Upadacitinib API. This can be crucial for pharmaceutical companies seeking reliable suppliers to meet the growing demand for Upadacitinib-based UC treatments.
Considering Upadacitinib API sourcing, it’s essential to conduct thorough research and due diligence to select a qualified manufacturer. Qingmu’s experience, commitment to quality, and global reach position them as a potential partner for pharmaceutical companies developing Upadacitinib-based medications for UC.