Understanding the Chemical Properties of Rotigotine API
Rotigotine API (Active Pharmaceutical Ingredient) is a vital component in the drug Neupro®, used to treat Parkinson’s disease and restless legs syndrome. Understanding its chemical properties is crucial for its safe and effective use. This article delves into the synthesis, production process, applications, and quality control measures employed by Qingmu for Rotigotine API.
Synthesis of Rotigotine API
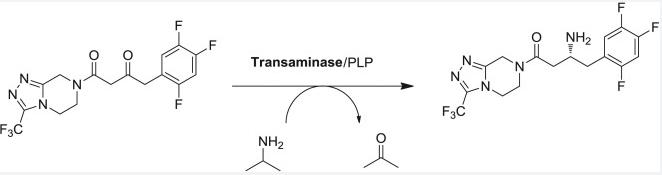
The synthesis of Rotigotine API is a complex process, but understanding the general steps involved can provide valuable insight. While the specifics are often trade secrets, here’s a breakdown of the potential involved:
1. Starting Materials:
N-(n-Propyl)-2-cyanoacetamide: This key component establishes the core structure of Rotigotine. Obtaining it with the desired chirality is crucial. Chiral molecules exist in mirror-image forms, and only one form (enantiomer) possesses the desired pharmacological activity for Rotigotine. Several methods can achieve this, including:
- Asymmetric Synthesis: Utilizing chiral catalysts or reagents to selectively synthesize the desired enantiomer.
- Resolution: Racemic synthesis (producing a mixture of both enantiomers) followed by separation techniques to isolate the specific enantiomer needed for Rotigotine.
- 2-Thiopheneacetic Acid: This molecule contributes the characteristic thiophene ring to Rotigotine’s structure. It’s likely commercially available or synthesized through established methods.
2. Functional Group Transformations:
- Amide Condensation: N-(n-Propyl)-2-cyanoacetamide reacts with 2-thiopheneacetic acid to form a new amide bond, linking the two core fragments. This reaction might involve dehydrating agents or specific catalysts to enhance efficiency.
- Nitrile Reduction: The cyano group (C≡N) on the initial N-(n-Propyl)-2-cyanoacetamide needs conversion to a primary amine (H2N-). This likely involves reduction using appropriate reducing agents like hydrogen gas in the presence of a catalyst.
3. Coupling Reactions:
Reductive Amination: The primary amine obtained from the previous step condenses with a carbonyl group (C=O) to form the final amine linkage in the Rotigotine molecule. The carbonyl group could come from various sources, depending on the specific synthetic route.
4. Purification:
After the final coupling reaction, the reaction mixture will contain Rotigotine API along with various byproducts and unreacted starting materials. Rigorous purification steps are essential to isolate pure Rotigotine. These might involve:
- Solvent Extraction: Utilizing the differing solubility of Rotigotine in various solvents to separate it from impurities.
- Chromatography: Passing the mixture through a column packed with a specific stationary phase to separate components based on their interaction with the stationary phase and the mobile solvent.
- Crystallization: Recrystallization from a suitable solvent can further enhance the purity of Rotigotine API by exploiting its tendency to form crystals with a defined structure.
Production Process of Rotigotine API

The production process of Rotigotine API likely follows these key stages:
Stage 1: Large-Scale Reaction
Imagine a scientific symphony. In this first act, chemists meticulously translate the lab-scale synthesis of Rotigotine API into a grand production. Here, colossal reactors replace beakers, and precise control systems ensure consistent quality. The chosen synthetic route, whether acylation or esterification, plays out on a grand scale, meticulously combining starting materials under controlled conditions like temperature, pressure, and reaction time.
Stage 2: Crystallization
The maestro of this chemical symphony now orchestrates the formation of pure Rotigotine API crystals. The reaction mixture, likely containing the desired molecule alongside impurities, undergoes a transformation. Techniques like controlled cooling or evaporation might be employed to nudge the Rotigotine API molecules out of solution, forming well-defined crystals. This process is crucial for isolating the therapeutic molecule from unwanted byproducts.
Stage 3: Filtration and Drying
The curtain rises on Act 3, where the separation of the crystals takes center stage. Sophisticated filtration techniques, like rotary filtration or pressure filtration, come into play. Imagine a giant sieve meticulously separating the precious Rotigotine API crystals from the leftover liquid, which might contain unwanted materials. Finally, meticulous drying, perhaps using techniques like vacuum drying or fluidized bed drying, ensures the complete removal of solvents and residual moisture. This dry, crystalline Rotigotine API is now a step closer to becoming a life-changing medication.
Stage 4: Packaging and Storage
The final act focuses on safeguarding the integrity of the Rotigotine API. Stringent quality control measures ensure the final product meets all purity and potency specifications. The API is then carefully packaged in airtight containers under controlled conditions like temperature and humidity to prevent degradation and maintain its effectiveness. This ensures that the Rotigotine API reaches patients in a form that delivers optimal therapeutic benefit.
Application Research of Rotigotine API
While Rotigotine API has established itself as a valuable treatment for Parkinson’s disease (PD) and restless legs syndrome (RLS), its potential extends far beyond these initial applications. Ongoing research delves into the exciting possibilities of Rotigotine for various neurological conditions. This article explores these promising areas of investigation:
1. Dosage Optimization:
Current treatment regimens for PD and RLS with Rotigotine involve a “one-size-fits-all” approach. However, research is actively exploring personalized medicine strategies. This involves tailoring Rotigotine dosage based on individual patient factors like disease severity, metabolism, and response to treatment. Optimizing dosage can potentially:
- Enhance efficacy: By delivering the most effective dose for each patient, symptoms can be controlled more effectively.
- Reduce side effects: Minimizing unnecessary medication can potentially lessen the occurrence of unwanted side effects.
- Improve patient compliance: A personalized approach can lead to a more manageable treatment plan, encouraging patients to adhere to their medication schedule.
2. Combination Therapies:
Rotigotine’s effectiveness can be further amplified by exploring combination therapies. This involves pairing Rotigotine with other medications targeting different aspects of the same neurological condition. For instance, in PD, Rotigotine could be combined with drugs that increase dopamine levels or address specific motor symptoms. Similarly, for RLS, Rotigotine might be used alongside medications that improve sleep quality or address nerve pain.
The potential benefits of combination therapy include:
- Synergistic effects: Combining medications can create a synergistic effect, where the combined action is greater than the sum of their individual effects.
- Broader symptom management: By targeting different aspects of the disease, combination therapy can offer a more comprehensive approach to managing symptoms.
- Slower disease progression: In some cases, combining medications can potentially slow down the progression of the underlying neurological condition.
3. New Formulations:

The current delivery method for Rotigotine is primarily through transdermal patches. However, researchers are exploring the development of new formulations with different delivery methods. Some potential areas of exploration include:
- Oral formulations: This could offer a more convenient option for patients who struggle with applying or keeping patches on.
- Injectable formulations: This could provide a faster and more targeted delivery of Rotigotine, potentially beneficial for acute symptom management.
- Nasal sprays or inhalers: These formulations could offer a faster-acting option compared to patches and might be particularly useful for breakthrough symptoms.
Developing new formulations can improve patient adherence, and treatment efficacy, and potentially address limitations associated with the current patch delivery system.
How Qingmu Do Quality Control of Rotigotine API?

To ensure the safety and efficacy of their Rotigotine API, Qingmu Pharmaceutical likely employs stringent quality control measures throughout the production process. These include:
- Starting Material Testing: Analyzing the purity and chirality of starting materials.
- In-Process Controls: Monitoring reactions at various stages to ensure they proceed as planned.
- Final Product Testing: Performing a battery of tests on the final Rotigotine API to confirm its chemical identity, purity, potency, and absence of contaminants.
- Stability Testing: Evaluating the stability of the Rotigotine API under different storage conditions.
By adhering to these rigorous quality control measures, Qingmu can ensure its Rotigotine API meets the highest standards for pharmaceutical use.
Qingmu is always your trusted partner in API manufacturing!