Synthesis Methods of Lenvatinib Mesylate API
Lenvatinib mesylate, a mesylate salt form of lenvatinib, is an oral multi-targeted tyrosine kinase inhibitor used primarily in the treatment of various cancers, including renal cell carcinoma, hepatocellular carcinoma, and thyroid cancer. As a complex organic molecule, its synthesis involves multiple steps, each crucial for ensuring the desired yield, purity, and overall quality of the final product. Understanding the synthesis methods of lenvatinib mesylate API is essential for both academic researchers and lenvatinib manufacturers aiming to optimize production processes.
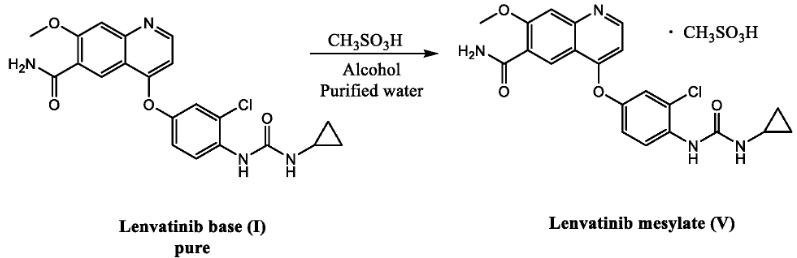
Common Synthetic Route of Lenvatinib Mesylate API
The synthesis of lenvatinib mesylate API involves a multi-step sequence of organic reactions. While the specific details of these routes may be protected by patents, scientific literature generally describes two main approaches:
Route 1: Starting from 4-Amino-3-chlorophenol and Chlorobenzoic Ester
This route begins with the condensation of 4-amino-3-chlorophenol and chlorobenzoic ester, followed by an amine hydrolysis step to generate a urea intermediate. Finally, this intermediate undergoes a nucleophilic substitution reaction with 4-chloro-7-methoxyquinolin-6-amide under basic conditions to afford Lenvatinib Mesylate.
Route 2: Utilizing N,N’-Carbonyldiimidazole
An alternative approach involves the reaction of N,N’-carbonyldiimidazole with cyclopropylamine to form an intermediate. This intermediate is then coupled with another molecule (compound 7) to yield Lenvatinib Mesylate, potentially with higher yields compared to route 1.
These are just two examples, and other synthetic routes for Lenvatinib Mesylate may exist. The choice of route depends on various factors, such as the availability of starting materials, reaction efficiency, and overall cost-effectiveness.
Key Intermediates and Their Role
The synthesis of lenvatinib mesylate involves several critical intermediates, each playing a pivotal role in the overall process. These intermediates often contain functional groups that require careful manipulation to achieve the desired product. Protecting groups are frequently employed to safeguard reactive sites during synthetic transformations. For instance, protecting the amine group in an intermediate might be necessary to prevent unwanted side reactions.
The successful synthesis of Lenvatinib mesylate hinges on the efficient and selective formation of these intermediates. Impurities or side products generated during intermediate synthesis can significantly impact the overall yield and purity of the final product. Therefore, robust purification techniques are essential to isolate the desired intermediates in high purity.
Critical Techniques in Lenvatinib Mesylate API Synthesis
Choosing the appropriate synthesis route is crucial for optimizing yield, purity, and overall efficiency. The selection process involves evaluating various factors such as the availability of starting materials, cost-effectiveness, reaction conditions, and environmental impact. Optimization of these routes can lead to significant improvements in the overall synthesis process, ensuring that the final product meets the required standards for medical use.
1. Reaction Condition Optimization
The optimization of reaction conditions, including temperature, pressure, solvent choice, and catalyst selection, plays a vital role in enhancing conversion rates and product selectivity. For instance, certain steps in the synthesis may require low temperatures to increase yield and reduce side reactions. Solvents must be chosen based on their ability to dissolve reactants and facilitate the desired reactions without causing degradation or unwanted side reactions.
2. Purification of Intermediates
Purifying intermediates is essential to ensure the success of subsequent reactions. Techniques such as column chromatography, crystallization, and extraction are commonly used to isolate and purify intermediates. Each technique must be carefully chosen based on the properties of the intermediate, such as its solubility and stability. Efficient purification minimizes impurities, leading to higher quality and yield of the final product.
3. Structural Confirmation of the Final Product
Confirming the structure of the final product is critical for verifying the success of the synthesis process. Analytical techniques such as nuclear magnetic resonance (NMR) and high-resolution mass spectrometry (HRMS) are employed to ensure that the synthesized compound matches the expected structure. These techniques provide detailed information about the molecular structure, helping to identify any deviations or impurities that may be present.
Organic Reactions Used in Lenvatinib Mesylate API Synthesis
- Substitution reactions, including electrophilic and nucleophilic substitutions, are commonly used in the synthesis of lenvatinib mesylate API. These reactions involve the replacement of one atom or group with another, allowing for the introduction of specific functional groups necessary for the desired product.
- Condensation reactions, which involve the combination of two or more molecules with the loss of a small molecule such as water, are essential for forming larger, more complex structures. These reactions are particularly useful for forming peptide and ether bonds.
- Chlorination reactions, where a hydrogen atom is replaced by a chlorine atom, increase the reactivity of the molecule and prepare it for further functionalization. These reactions are crucial for introducing reactive sites that facilitate subsequent transformations.
- Amidation reactions, involving the reaction of amines with acids or anhydrides to form amides, are key steps in the synthesis of Lenvatinib Mesylate. These reactions are fundamental in the construction of peptide bonds and other nitrogen-containing compounds.
- The formation of amide bonds is a core step in the synthesis of lenvatinib mesylate API. This process typically involves the reaction of carboxylic acids or their derivatives with amines, forming stable amide bonds that are integral to the structure of the final product.
- The final step in the synthesis of Lenvatinib Mesylate involves salification, where the free base of lenvatinib reacts with methanesulfonic acid to form the mesylate salt. This step enhances the stability and solubility of the drug, making it suitable for pharmaceutical applications.
Optimizing Reaction Conditions for Efficient Synthesis
The success of lenvatinib mesylate synthesis hinges on meticulously controlled reaction conditions. Here are some critical factors that chemists consider:
- Temperature: Reaction temperature significantly impacts reaction rates and product distribution. Lenvatinib Mesylate synthesis may involve steps requiring low temperatures to favor the formation of the desired product and minimize side reactions.
- Pressure: Pressure can influence reaction equilibria and reaction rates. In some steps, applying pressure might be necessary to achieve optimal conversion.
- Solvent Selection: The choice of solvent plays a crucial role in solubility, reaction rates, and selectivity. Selecting the appropriate solvent ensures efficient mixing of reactants, promotes the desired reaction pathway, and minimizes unwanted side reactions.
- Catalyst Selection: Catalysts can accelerate reaction rates and improve efficiency. Different catalysts may be employed throughout the Lenvatinib Mesylate synthesis to optimize reaction outcomes for specific steps.
- Reaction Time: Allowing sufficient reaction time is essential for complete conversion of starting materials into the desired product. However, excessively long reaction times can lead to decomposition or degradation of the product.

Quality Control Measures for Ensuring Purity
Stringent quality control measures are implemented throughout the lenvatinib mesylate API synthesis process to guarantee the final product’s purity, safety, and efficacy. Here are some common analytical techniques employed:
- Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR is a powerful technique for determining the structure of organic compounds. It provides detailed information about the molecule’s functional groups and connectivity. By comparing the NMR spectrum of the synthesized Lenvatinib Mesylate with the reference spectrum, chemists can confirm the product’s identity and purity.
- Mass Spectrometry (MS): MS is used to determine the molecular weight of a compound. By analyzing the mass-to-charge ratio of ions, MS can confirm the molecular formula of Lenvatinib Mesylate and detect impurities with different molecular weights.
- High-Performance Liquid Chromatography (HPLC): HPLC is a chromatographic technique that separates and analyzes compounds based on their polarity. It is used to determine the purity of Lenvatinib Mesylate by comparing the retention time of the sample with that of a reference standard.
- Infrared Spectroscopy (IR): IR spectroscopy provides information about the functional groups present in a molecule. By comparing the IR spectrum of the synthesized Lenvatinib Mesylate with the reference spectrum, chemists can confirm the presence of expected functional groups and identify potential impurities.
- Elemental Analysis: Elemental analysis determines the elemental composition of a compound. By comparing the calculated elemental composition of Lenvatinib Mesylate with the experimentally determined values, chemists can verify the molecular formula and detect the presence of impurities.
In addition to these analytical techniques, other quality control measures include chiral purity analysis (for chiral compounds), impurity profiling, and stability testing to ensure the long-term stability and safety of the drug product.

Conclusion
The synthesis of lenvatinib mesylate API is a complex process involving multiple steps and critical techniques. From selecting the appropriate synthesis route to optimizing reaction conditions and ensuring quality control, each stage plays a vital role in producing a high-quality final product. Understanding these synthesis methods is essential for both academic researchers and lenvatinib manufacturers aiming to optimize production processes and ensure the availability of this important cancer treatment. Future advancements in synthesis techniques and industrial applications promise to further improve the efficiency, yield, and quality of lenvatinib mesylate API.