Ruxolitinib: From API to Breakthrough Vitiligo Treatment
Ruxolitinib, a potent Janus kinase (JAK) inhibitor, has carved its niche in treating diverse medical conditions ranging from myelofibrosis to atopic dermatitis. Its journey, however, wasn’t solely confined to the realm of systemic applications. In a recent landmark development, ruxolitinib phosphate took the form of a topical cream, heralding a new era for vitiligo management. This article delves into the intricate world of ruxolitinib, exploring its Active Pharmaceutical Ingredient (API) intricacies, manufacturer landscape, and its transformative role in vitiligo therapy.
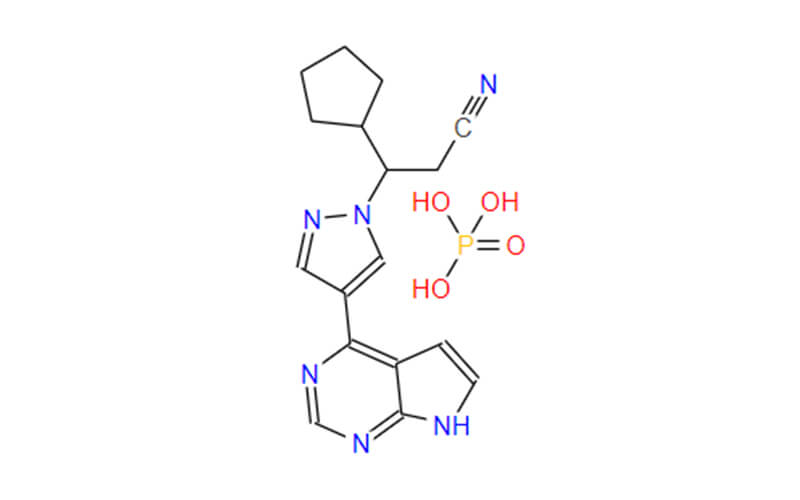
Ruxolitinib API: the Core of Therapeutic
The Active Pharmaceutical Ingredient (API) of ruxolitinib, the cornerstone of its therapeutic efficacy, takes the form of ruxolitinib phosphate. This crystalline salt, strategically chosen for its optimized properties, surpasses its free base counterpart in both aqueous solubility and stability. These enhanced characteristics are pivotal for efficient drug formulation and topical delivery, paving the way for its groundbreaking application in vitiligo treatment.
The intricate journey of ruxolitinib phosphate from raw material to its final API form is a meticulously orchestrated multi-step process. Each stage, from initial synthesis through rigorous purification and characterization, is subject to stringent quality control measures. Adherence to stringent regulatory standards, such as cGMP (current Good Manufacturing Practice) guidelines, ensures the API’s unwavering purity, potency, and batch-to-batch consistency. This unwavering commitment to quality translates to safe and efficacious medications reaching patients, underpinning the very foundation of successful therapeutic interventions.
The synthesis of ruxolitinib phosphate itself involves a series of precisely controlled chemical reactions. Specific precursors undergo targeted transformations, employing specialized reagents and catalysts under controlled temperature, pressure, and pH conditions. Subsequent purification steps utilize techniques such as chromatography and crystallization to remove impurities and isolate the desired API. Stringent analytical testing at each stage, employing advanced spectroscopic and chromatographic techniques, ensures the API meets the stringent quality specifications outlined in regulatory guidelines.
Characterization of the final ruxolitinib phosphate API delves deeper, evaluating its physical and chemical properties. Particle size distribution, surface area analysis, and hygroscopicity are crucial parameters assessed, directly impacting the API’s handling, stability, and subsequent formulation into topical creams or other dosage forms. Furthermore, comprehensive impurity profiling ensures the absence of potential contaminants that could impact the drug’s safety and efficacy.
Ruxolitinib Mechanisms of Action for Vitiligo Treatment
Vitiligo, a chronic autoimmune disorder characterized by melanocyte destruction and consequent patchy depigmentation, has long posed a significant therapeutic challenge. Its intricate pathogenesis, involving aberrant T cell activation and pro-apoptotic signaling cascades, effectively evaded the control offered by conventional treatments. However, the arrival of ruxolitinib, a highly targeted Janus kinase (JAK) inhibitor, has ignited a beacon of hope for vitiligo patients.
The JAK-STAT signaling pathway plays a central role in orchestrating the pro-apoptotic cascade responsible for melanocyte demise in vitiligo. Ruxolitinib, with its exquisite selectivity towards JAK1 and JAK2, directly disrupts this signaling axis. By binding to the ATP-binding pocket of these kinases, it effectively halts the phosphorylation of downstream STAT (signal transducer and activator of transcription) proteins, thereby impeding the expression of genes involved in melanocyte apoptosis. This targeted intervention serves as a shield, protecting vulnerable melanocytes from the autoimmune onslaught.
The therapeutic potential of this JAK-mediated melanoprotection has been vividly demonstrated in clinical trials. Topical ruxolitinib cream has shown promising efficacy in vitiligo patients, with statistically significant repigmentation observed after 24 weeks of treatment. Studies report improvements in both facial and non-facial vitiligo, highlighting the broad applicability of this novel approach. The observed repigmentation extends beyond subjective patient assessments, evidenced by quantitative measures such as the Vitiligo Area Scoring Index (VASI) and Melanin Index.
Furthermore, ruxolitinib’s targeted action offers potential advantages over traditional vitiligo therapies. Unlike corticosteroids, which often carry the risk of skin atrophy and other side effects, ruxolitinib’s topical application exhibits a favorable safety profile. Additionally, its specific targeting of the JAK-STAT pathway holds promise for long-term efficacy and potentially reduced risk of recurrence, offering a potentially transformative shift in vitiligo management.
Qingmu: Assuring Ruxolitinib API Quality and Availability
Within the global landscape of ruxolitinib Active Pharmaceutical Ingredient (API) manufacturers, Qingmu stands as a trusted partner, committed to delivering unwavering quality and reliable supply. Our unwavering commitment to excellence extends across every facet of the API production process, from meticulous synthesis to rigorous quality control, ensuring the highest standards are met for every batch.
Unwavering Quality: A Cornerstone of Qingmu’s Philosophy
Qingmu’s dedication to quality begins at the very foundation of our operations. We employ a robust and well-established cGMP (current Good Manufacturing Practice) compliant manufacturing system, ensuring adherence to the most stringent international regulatory standards. This rigorous framework governs every stage of the production process, from initial raw material sourcing to final product packaging and distribution.
Expertise in Ruxolitinib API Synthesis
Leveraging our deep scientific expertise and cutting-edge technology, Qingmu has established a highly optimized and scalable synthesis process for ruxolitinib phosphate API. Meticulous control of reaction conditions, coupled with the utilization of advanced purification techniques, guarantees the consistent production of high-purity, crystalline ruxolitinib phosphate that meets the most demanding specifications.
Stringent Quality Control Measures
Qingmu’s commitment to quality extends beyond the production floor. We employ a comprehensive multi-tiered quality control system, encompassing both in-process and finished product testing. This rigorous regime utilizes state-of-the-art analytical instruments, including High-Performance Liquid Chromatography (HPLC), Mass Spectrometry (MS), and Nuclear Magnetic Resonance (NMR) spectroscopy, to meticulously assess the API’s purity, potency, and consistency. Each batch undergoes rigorous testing against established quality benchmarks, ensuring complete compliance with pharmacopoeial standards and client specifications.
Reliable Supply: A Global Reach
Qingmu understands the critical importance of reliable API supply for pharmaceutical manufacturers. To ensure uninterrupted access to ruxolitinib API, we have established a robust and geographically diverse supply chain. Our strategically located manufacturing facilities, coupled with a reliable network of distribution partners, guarantee timely and efficient delivery to customers worldwide. This commitment to global reach and responsiveness ensures seamless integration into your production processes, minimizing potential disruptions and delays.

Qingmu: Your Trusted Partner in Ruxolitinib API Manufacturing
By prioritizing unwavering quality, robust expertise, and a dedication to reliable supply, Qingmu has established itself as a leading manufacturer of ruxolitinib API. We are committed to providing our partners with the confidence and assurance they need to develop and deliver safe, efficacious, and life-changing treatments for patients around the world.
If you are looking for a reliable pharmaceutical API manufacturer, Qingmu is waiting for you.