Qingmu’s Quality Control of Nilotinib API
Nilotinib Hydrochloride (Nilotinib HCL) is a crucial medication used in the treatment of chronic myeloid leukemia (CML). As with any pharmaceutical product, maintaining the utmost quality of Nilotinib HCL is paramount for patient safety and treatment efficacy. This article explores the significance of quality control in Nilotinib HCL API (Active Pharmaceutical Ingredient) and how Qingmu prioritizes stringent measures throughout the manufacturing process.
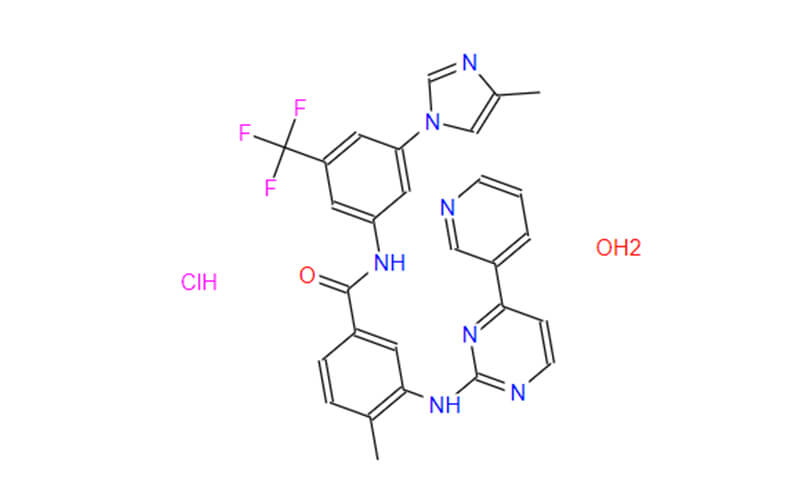
Introduction to Nilotinib HCL
In simple terms, Nilotinib HCL is the active ingredient that makes these medications effective. Think of it as the key component that fights cancer cells in the body.
Nilotinib HCL works by targeting a protein called BCR-ABL, which is responsible for the growth and spread of cancerous cells. By inhibiting this protein, Nilotinib HCL helps to prevent the proliferation of these harmful cells, thereby controlling the progression of the disease. This targeted approach not only makes the medication effective but also minimizes damage to healthy cells, reducing potential side effects.
As the foundational element of Nilotinib medications, Nilotinib HCL is crucial. It undergoes rigorous quality control processes to ensure its purity and effectiveness. Once thoroughly tested and validated, this compound is used to produce the final medication that doctors prescribe to patients. This ensures that each dose patients take is both safe and potent, providing the best possible treatment for managing their leukemia.
Importance of Quality Control in Nilotinib HCL API

Maintaining consistent quality in Nilotinib HCL API is essential for several reasons:
- Patient Safety: Impurities or inconsistencies in the API can lead to adverse reactions or reduced effectiveness of the medication. Even minor deviations in quality can have serious consequences. For instance, trace amounts of heavy metals or residual solvents can cause organ damage or allergic reactions. In the case of Nilotinib HCL, strict quality control ensures patients receive a pure medication, minimizing the risk of these potential complications.
- Treatment Efficacy: High-quality Nilotinib HCL ensures patients receive the precise dosage for optimal therapeutic effect. Nilotinib HCL targets a specific protein in cancer cells. The medication’s effectiveness relies on its potency and consistent delivery of the therapeutic dose. Rigorous quality control procedures guarantee that each Nilotinib HCL capsule contains the exact amount of active ingredient needed to achieve the desired outcome. Fluctuations in the API’s purity or concentration can significantly impact a patient’s treatment course.
- Regulatory Compliance: Pharmaceutical manufacturers must adhere to strict guidelines set forth by regulatory bodies like the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA). These regulations ensure the safety and efficacy of medications. Quality control measures implemented by manufacturers like Qingmu play a vital role in guaranteeing compliance with these regulations. Failing to meet these standards can result in production delays, product recalls, and even legal repercussions.
Generally speaking, maintaining the highest quality standards in Nilotinib HCL API is non-negotiable. It safeguards patient well-being, optimizes treatment outcomes, and ensures adherence to regulatory requirements. By prioritizing meticulous quality control measures, Qingmu fosters trust and delivers a reliable Nilotinib HCL API for successful patient care.
Qingmu’s Quality Control Measures for Nilotinib API

Qingmu prioritizes unmatched quality in Nilotinib HCL API production. Our commitment is reflected in a multi-layered quality control program implemented throughout the entire manufacturing process. Here’s a closer look at some of the key measures ensuring the consistent delivery of exceptional Nilotinib HCL:
- Rigorous Raw Material Selection: Qingmu maintains a stringent vendor qualification process. Only suppliers who meet their exacting standards and can consistently provide materials with impurity levels below established thresholds are approved. This ensures a strong foundation for quality right from the very first step.
- Multi-stage Quality Checks: Nilotinib HCL undergoes a battery of tests at various stages of production. These tests employ a combination of classical pharmacopoeial methods and cutting-edge analytical techniques.
- Classical methods like chromatography and gravimetric analysis ensure the API conforms to established quality parameters, such as identity, purity, potency, and dissolution rate.
- Advanced analytical techniques like mass spectrometry and nuclear magnetic resonance (NMR) delve deeper, providing an even more precise characterization of the Nilotinib HCL molecule. This allows for the detection and quantification of even trace impurities that might escape classical methods.
- Statistical Process Control (SPC): Qingmu utilizes SPC to continuously monitor critical parameters throughout the manufacturing process. SPC involves collecting and analyzing real-time data to identify any trends or deviations from specifications. This proactive approach allows for early detection and rectification of potential issues, ensuring consistent quality throughout production batches.
- Stability Testing: Qingmu conducts comprehensive stability testing on Nilotinib HCL API to assess its physical, chemical, and microbiological stability over time under various storage conditions. This data is crucial for determining shelf life and establishing appropriate storage and handling recommendations to guarantee the quality and efficacy of Nilotinib HCL throughout its intended use.
- cGMP Compliance: Qingmu’s manufacturing facilities adhere to Current Good Manufacturing Practices (cGMP) as outlined by regulatory bodies. cGMP regulations encompass a wide range of quality control practices, ensuring that all aspects of production, from raw materials to finished products, meet the highest quality standards.
By implementing these meticulous quality control measures, Qingmu ensures that every batch of Nilotinib HCL API they produce meets the strictest quality requirements. This unwavering commitment translates to patient safety, consistent treatment efficacy, and peace of mind for pharmaceutical companies who choose Qingmu as their trusted Nilotinib HCL supplier.
Choosing Qingmu as Your Nilotinib Supplier

By selecting Qingmu as your Nilotinib supplier, you can be confident that you are receiving a product manufactured with the highest quality standards. Qingmu’s commitment to quality control ensures patient safety, treatment efficacy, and regulatory compliance.
Partner with Qingmu for your Nilotinib HCL API needs and experience the difference that unwavering quality commitment brings.