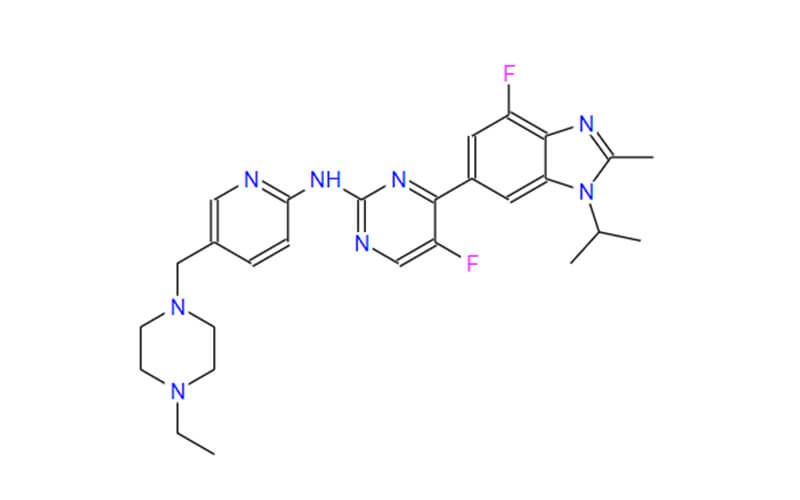
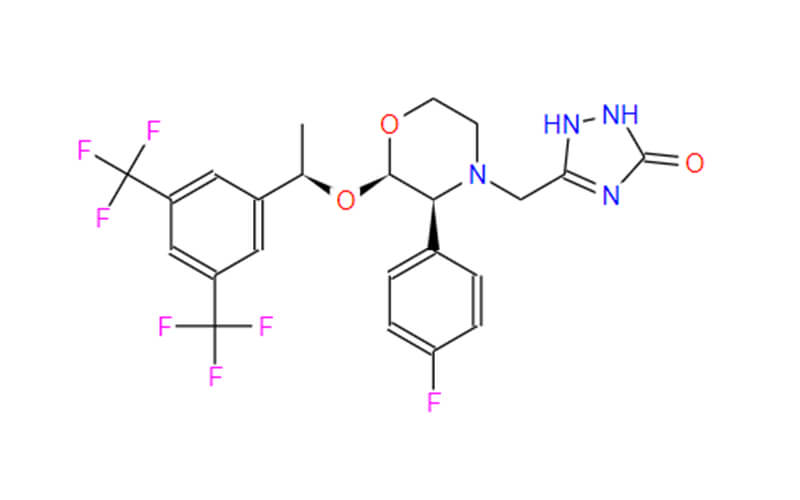
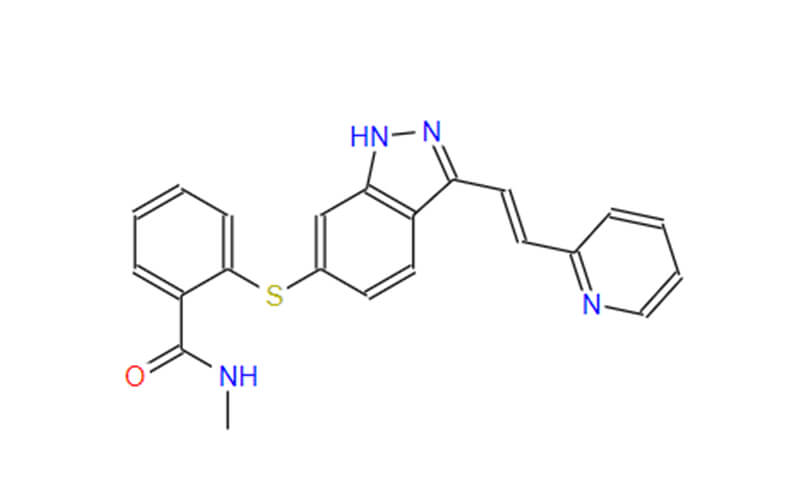
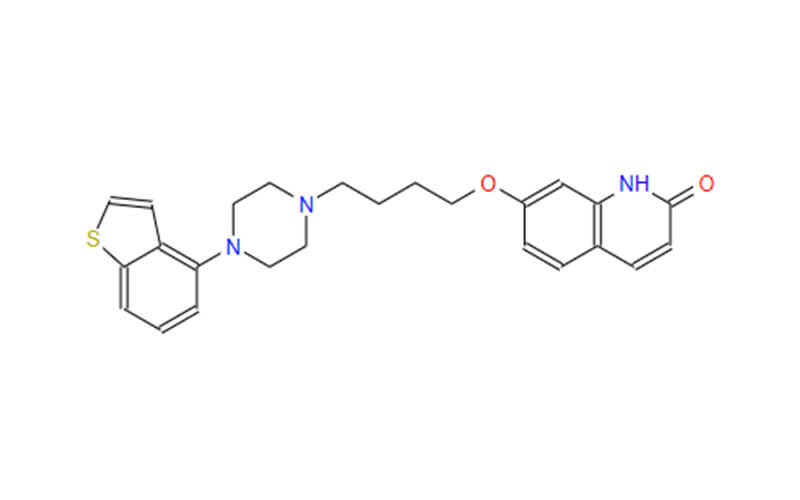
Human APIs
Products
Our Products
Abemaciclib API
CAS No.: 1231929-97-7
Aprepitant API
CAS No.: 170729-80-3
Axitinib API
CAS No.: 319460-85-0
Bisoprolol Fumarate API
CAS No.: 104344-23-2
Brexpiprazole API
CAS No.: 913611-97-9
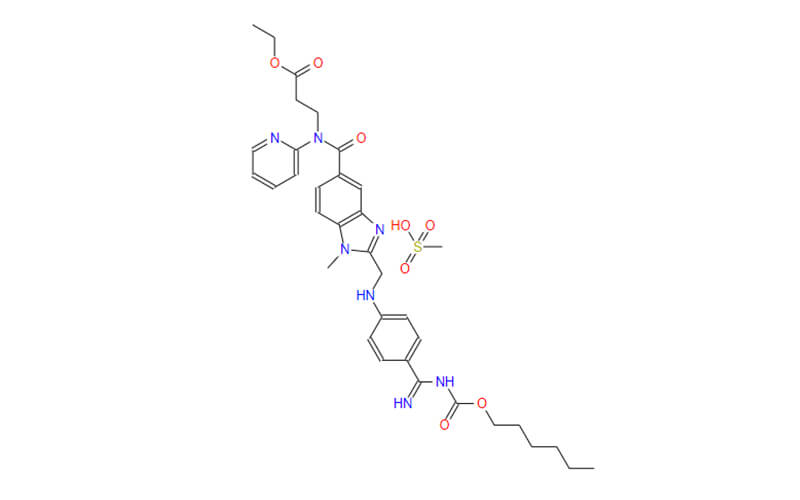
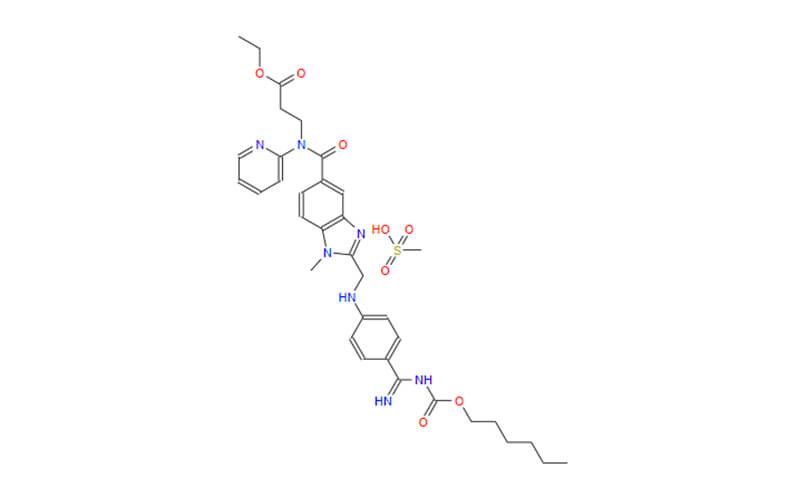
Dabigatran Etexilate Mesylate API
CAS No.: 872728-81-9
Dabigatran Etexilate Mesylate Pellets
CAS No.: 872728-81-9
Fruquintinib API
CAS No.: 1194506-26-7
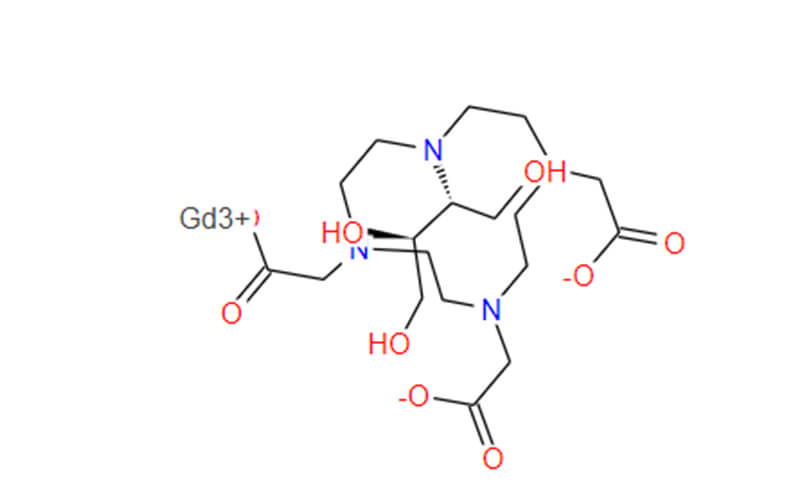
Gadobutrol API
CAS No.: 138071-82-6
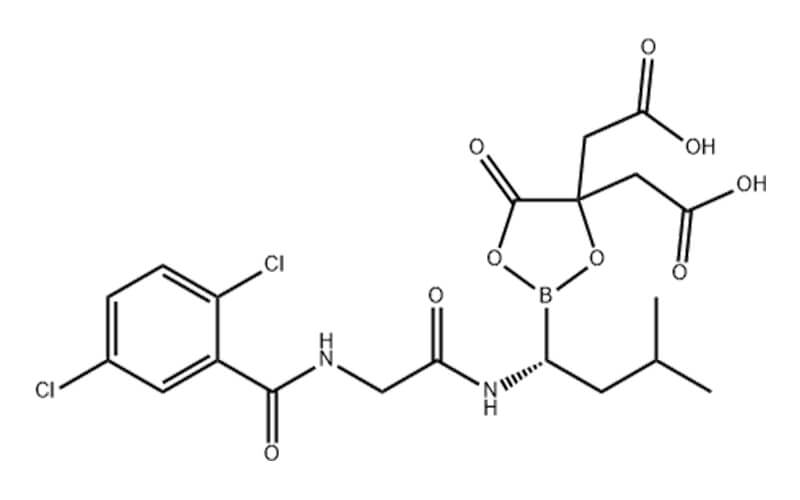
Ixazomib Citrate API
CAS No.: 1239908-20-3
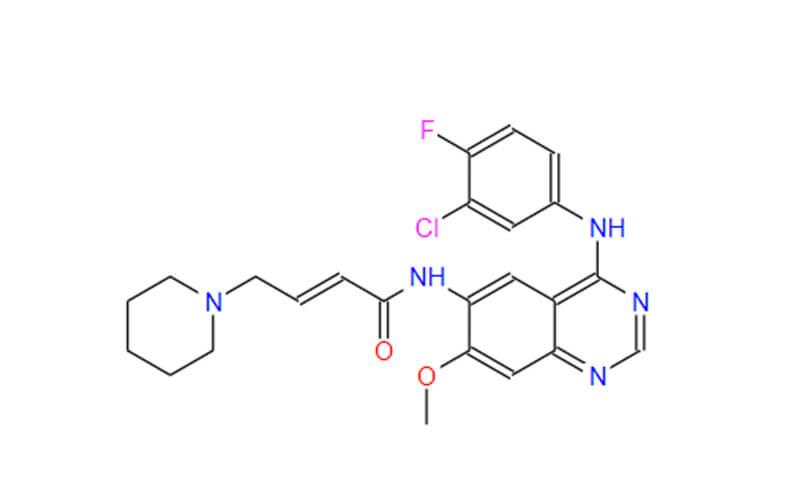
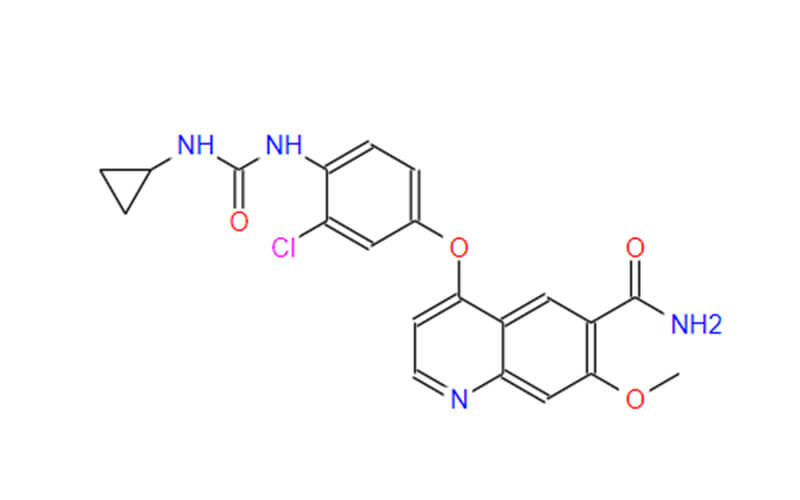
Lenvatinib Mesilate API
CAS No.: 417716-92-8
Lurbinectedin API
CAS No.: 417716-92-8
Qingmu Pharmaceutical is dedicated to the production of Bulk Drug API (Active Pharmaceutical Ingredients). Our manufacturing facility holds the coveted approvals of both the US FDA and the Chinese NMPA (GMP-PLANT), equipped with cutting-edge technology for the development and refinement of production processes. Our Human APIs are known for their exceptional cost-effectiveness, and we proudly manufacture over 30 different Pharma APIs.
With more than a decade of expertise, Qingmu Pharmaceutical stands at the forefront of Human APIs manufacturing in China. We firmly believe that ensuring access to quality healthcare is not a privilege but an integral part of life. We take pride in delivering globally acclaimed Human Health APIs at competitive prices, ensuring the supply of Pharma APIs on a worldwide scale.
Remarks: According to laws and regulations of People’s Republic of China, relevant information of Schedule II Controlled Substance APIs is not released here.
Disclaimer: The product that is still under patent protection can only be used in research and development, not for commercial purpose.