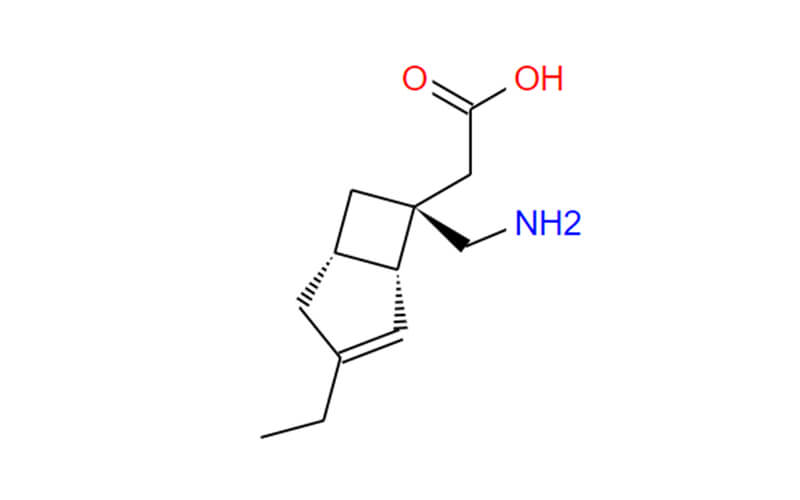
Mirogabalin Besilate API
| CAS No. | 1138245-13-2 |
| Therapeutic Category | Anti-epileptic |
| Technology | Synthetic |
| Dosage Form | Oral Solids |
| Innovator Brand | Tarlige(USA) |
| Registration Status | R&D |
| Polymorph | N/A |
| GMP | FDA(USA)/NMPA(China)/MFDS(Korea) approved |
| EHS | ISO 14001 & 45001 certified |
Product Description
- Grade: Active Pharmaceutical Ingredient(API)
- Chemical Name:((1R,5S,6S)-6-(carboxymethyl)-3-ethylbicyclo[3.2.0]hept-3-en-6-yl)methanaminiumbenzenesulfonate;[(1R,5S,6S)-6-(Aminomethyl)-3-ethylbicyclo[3.2.0]heptChemicalbook-3-en-6-yl]aceticacidbenzenesulfonate;2-((1R,5S,6S)-6-(aminomethyl)-3-ethylbicyclo[3.2.0]hept-3-en-6-yl)aceticacidcompoundwithbenzenesulfonicacid(1:1)
- Molecular Formula: C12H19NO2 • C6H6O3S
- Molecular Weight: 367.46
- Specification: Enterprise Standard established according to ChP/USP/EP
- Appearance: Powder
- Total impurities: not more than 0.5%
- Purity: not less than 99%
- Residual Solvents: fully comply with ICH Q3C
- Mutagenic impurities: fully comply with ICH M8
- Nitrosamine assessment: available
- Particle size: regular grade or milling/sieving according to customer’s requirement.
- Storage: Room temperature
- Production capacity: R&D
- Standard Package: 1kg/bag, 5kg/bag, or according to the customer’s requirement
Applications of Mirogabalin API
- Mirogabalin is a calcium channel blocker with analgesic effects. It binds to the α2δ-1 and α2δ-2 subunits of voltage-dependent Ca2+ channels. Mirogabalin has potent and sustained analgesic effects (ED50 = 2.5 mg/kg) in rats with diabetes induced by streptozotocin (STZ; ). Mirogabalin does not inhibit activities associated with CNS adverse effects of analgesics, such as rotarod performance (ID50 = 9.4 mg/kg) or locomotor activity (ID50 = 43.9 mg/kg), at its effective dose. Formulations containing mirogabalin are in clinical trials for diabetic peripheral neuropathic pain.
Why Choose Us as Your Mirogabalin Manufacturer?
- Established according to ICH/USFDA/EU/JAPAN/China regulations and current GMP, Qingmu’s factory totally complies with environmental law in China, without the risk of relocating factories. A new API factory is under design and is predicted to be put into use in 2025.
- Qingmu’s team has rich experience in patent challenges on crystalline form & synthesis processes and also synthetic route development and scale-up & quality research.
- Qingmu’s lab is equipped with HPLC, GC, ICP-MS, GC-MS, CAD, microbalance, Malvern particle analyzer, etc. Qingmu passed the site inspection from NMPA(China), FDA(USA), and MFDS(Korea) as well as customer audits from Europe, USA, Japan, etc.
- As a China Mirogabalin supplier, Qingmu successfully exported products to more than 40 countries including Japan, USA, Germany, Spain, the Netherlands, Russia, South Korea, and Brazil.