Potential of Lenvatinib Mesylate in Liver Cancer Treatment
Liver cancer continues to pose a formidable challenge to global health, particularly in China, where incidence and mortality rates remain alarming. Recent statistics from the International Agency for Research on Cancer (IARC) reveal a staggering 410,000 new cases and 370,000 deaths attributed to liver cancer in 2020 alone within China. The intricate nexus of factors including hepatitis B and C infections, cirrhosis, non-alcoholic fatty liver disease, lifestyle decisions, and dietary contamination collectively fuel the pervasive and grave nature of liver cancer within the nation.
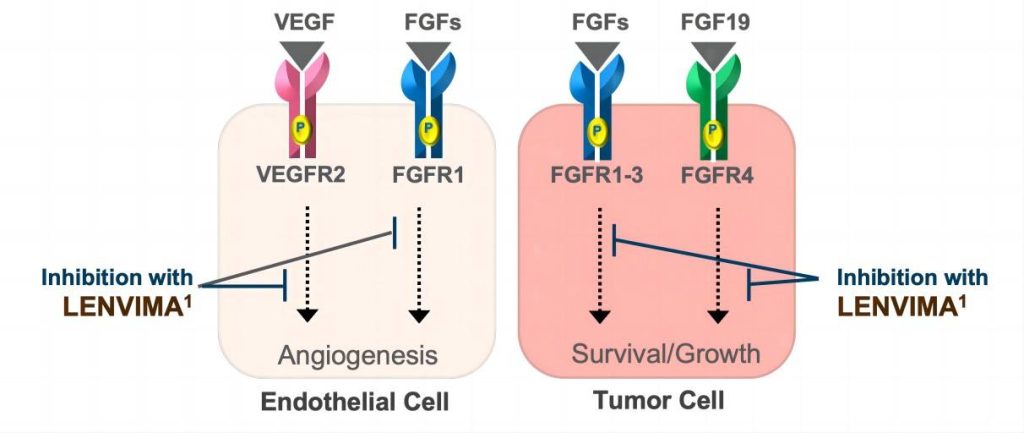
While various treatment modalities exist, including surgery, local ablation, interventional therapies, and traditional chemotherapy, the past decade has seen significant advancements in liver cancer drug development. Among the targeted therapies, Lenvatinib Mesylate, also known as Lenvatinib or lenvatinib API (Active Pharmaceutical Ingredient), has emerged as a crucial player. Lenvatinib, endorsed in China as of November 2018, serves as a multi-targeted receptor tyrosine kinase (RTK) inhibitor. It stands as the second primary first-line targeted therapy for liver cancer following Sorafenib.
What’s the Efficacy of Lenvatinib in Liver Cancer?
Lenvatinib has emerged as a promising advancement in the battle against liver cancer, particularly surpassing its predecessor, Sorafenib. The REFLECT study, a groundbreaking global trial involving Chinese patients, presented compelling evidence of its potential. Lenvatinib notably outperformed Sorafenib in terms of both overall and progression-free survival rates. Patients treated with Lenvatinib experienced a median overall survival of 15.0 months, compared to 10.2 months with Sorafenib, and their cancer progression was significantly delayed, with a median of 9.2 months versus 3.6 months with Sorafenib.
However, a cloud of uncertainty hangs over this promising therapy. Despite its strong showing in the REFLECT study, approximately 80% of liver cancer patients do not respond effectively to Lenvatinib treatment. This unresponsiveness poses a significant challenge for patients and clinicians alike.
A recent study published in Nature titled “EGFR activation limits response of liver cancer to lenvatinib” has shed light on potential mechanisms underlying this limited efficacy. This research provides invaluable insights that could lead to improved treatment strategies.
What’s the Factor of EGFR?
The study utilized cutting-edge CRISPR-Cas9 gene editing technology within a high-throughput functional screening system. Through this innovative approach, researchers made a significant breakthrough in understanding the dynamics of liver cancer cells in response to Lenvatinib treatment.
Their findings revealed that the knockout of the epidermal growth factor receptor (EGFR) notably heightened the sensitivity of liver cancer cells to Lenvatinib. This discovery sheds light on a crucial molecular pathway that influences the effectiveness of Lenvatinib in combating liver cancer.
Delving deeper into the molecular mechanisms at play, the researchers uncovered a fascinating sequence of events. Upon administration of Lenvatinib, which inhibits key receptor tyrosine kinases (RTKs) such as fibroblast growth factor receptor (FGFR), EGFR undergoes a feedback activation process. This activation triggers downstream signaling cascades, particularly involving PAK2-ERK5 signaling and the shared downstream MEK1/2-ERK1/2 pathway with FGFR.
This intricate feedback loop allows liver cancer cells to sustain robust survival and proliferative capabilities, even in the face of continuous Lenvatinib treatment. Such insights into the molecular intricacies of Lenvatinib resistance offer valuable clues for developing more effective therapeutic strategies against liver cancer.
Combination Therapy as A New Approach
Equipped with insights into EGFR‘s role in Lenvatinib resistance, researchers engineered a clever strategy: a dual-pronged assault. They paired Lenvatinib with Gefitinib, a drug specifically aimed at targeting EGFR. This tandem approach underwent rigorous testing in both mouse models and patient-derived tumors (PDXs), mirroring real-world conditions. The outcomes proved exceptionally promising.
This combined therapy delivered a formidable blow, notably reducing tumor sizes, particularly in cases exhibiting high EGFR expression. Yet, the narrative extends beyond mere tumor shrinkage. This dynamic combination also orchestrated a remarkable transformation within the tumor’s internal environment. It beckoned forth an army of cancer-fighting protagonists: natural killer (NK) cells and CD8-positive T cells, renowned for their tumor-suppressing prowess. Concurrently, the gatecrashers, tumor-associated macrophages notorious for dampening the immune response, were swiftly ousted. This shift in the tumor microenvironment implies that combination therapy not only directly assaults cancer cells but also amplifies the body’s innate immune defenses, orchestrating a dual assault against liver cancer.
This groundbreaking research sets the stage for a new era of liver cancer treatments harnessing the potential of combination therapy. By unraveling the intricate interplay between drugs and the tumor’s immune landscape, we can devise therapies that not only target the cancer itself but also unleash the body’s intrinsic defenses.
Ongoing Clinical Trials and Future Prospects
Fueled by the lab’s findings, Renji Hospital launched a Phase I clinical trial (NCT04642547) to test the real-world impact of the Lenvatinib-Gefitinib combo in advanced liver cancer. The initial results painted a hopeful picture – out of 12 patients with high EGFR expression who hadn’t responded to Lenvatinib alone, 4 saw their tumors shrink significantly. This early success paves the way for larger studies to confirm the potential of this powerful partnership.
But Lenvatinib’s potential goes beyond liver cancer. Its versatility is being explored in a web of promising studies across various solid tumors. One such collaboration pairs Lenvatinib with Trastuzumab to tackle liver cancer, while another teams it up with Pembrolizumab to fight malignancies like endometrial cancer, melanoma, and even triple-negative breast cancer. This impressive list extends to non-small cell lung cancer, renal cell carcinoma, head and neck cancers, bladder cancer, colorectal cancer, gastric cancer, glioblastoma, and ovarian cancer.
The future for Lenvatinib mesilate is brimming with possibilities. By understanding its intricate dance with other drugs and the tumor’s immune landscape, researchers are unlocking a treasure trove of treatment options. This, in turn, translates to a brighter future for patients facing a diverse range of cancers, offering them a powerful ally in their fight for life.
Summary
The liver cancer treatment landscape has seen significant progress thanks to targeted therapies like Lenvatinib. However, its limitations underscore the complexity of cancer biology. Identifying EGFR activation as a resistance factor not only reveals intricacies but also paves the way for deeper research and therapeutic breakthroughs.
Recent studies on combination therapies bring hope to overcoming resistance mechanisms and enhancing the immune response against liver cancer. Ongoing clinical trials exploring Lenvatinib’s potential in various solid tumors paint a promising picture for cancer treatment’s future.
Expectations for more innovative drugs and personalized approaches highlight the importance of collaboration between pharmaceutical companies, research institutions, and healthcare professionals. This synergy is crucial for advancing oncology, offering patients a wider range of effective treatments. Acknowledging the role of Lenvatinib’s manufacturer in this collaboration underscores the collective commitment to improving cancer care and accessibility.
References
Jin, H. et al. EGFR activation limits the response of liver cancer to lenvatinib. Nature 595, 730–734 (2021).