Ixazomib Citrate API in the Age of Biosimilars
The pharmaceutical landscape is continually evolving, with biologics and biosimilars playing pivotal roles in advancing treatment options for various diseases. Among these, Ixazomib Citrate API stands out for its significant impact in oncology, particularly in the treatment of multiple myeloma. As we delve into the age of biosimilars, understanding the dynamics between biologics, biosimilars, and Ixazomib Citrate API is crucial for healthcare professionals, researchers, and patients alike.
Biologics vs. Biosimilars
Biologics are intricate, large-molecule drugs derived from living organisms. These advanced medications have revolutionized the treatment landscape for a wide array of conditions, including cancers, autoimmune diseases, and chronic inflammatory disorders. The sophistication of biologics lies in their complexity and the intricate manufacturing processes required to produce them. These processes ensure their high efficacy, but they also contribute to the significant costs associated with biologic therapies, making them less accessible to many patients.
Biosimilars, however, offer a promising alternative. These drugs are highly similar to their reference biologics but are not exact replicas. Despite minor differences, biosimilars provide comparable efficacy, safety, and quality at a fraction of the cost of the original biologics. This cost-effectiveness stems from the ability to replicate the therapeutic effects of biologics without the need for the extensive and costly research and development processes that the original drugs underwent.
The development of biosimilars involves rigorous testing to ensure they match the reference biologics in terms of structure, function, and clinical performance. These evaluations include comprehensive analytical studies, preclinical trials, and clinical trials to confirm their similarity in efficacy and safety. Regulatory agencies, such as the FDA and EMA, have stringent guidelines to ensure that biosimilars meet the necessary standards for approval.
Mechanism of Action of Ixazomib Citrate API
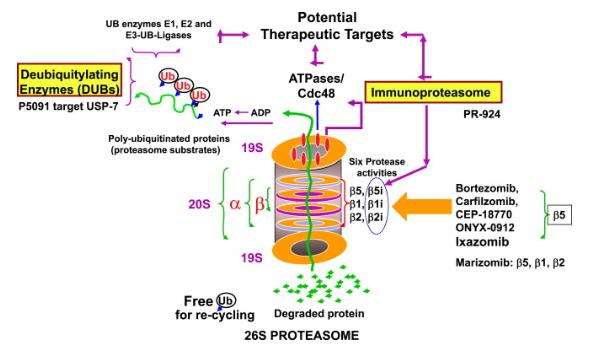
Ixazomib Citrate API belongs to a class of drugs called proteasome inhibitors. Proteasomes are cellular structures that function like a recycling center within cells. They are responsible for breaking down proteins that are no longer needed or are damaged. This breakdown process allows the cell to reuse the building blocks (amino acids) to create new proteins.
In Multiple Myeloma (MM), cancer cells often produce abnormal proteins. These abnormal proteins can accumulate inside the cancer cells, hindering their normal function and promoting their growth and survival. Ixazomib Citrate API works by specifically inhibiting a critical site within the proteasome, known as the chymotrypsin-like (CT-L) site. By blocking this site, Ixazomib Citrate API disrupts the proteasome’s ability to break down proteins.
This targeted inhibition leads to a buildup of these abnormal proteins within the cancer cells. As these abnormal proteins accumulate, they become toxic to the cancer cells, ultimately triggering a process called apoptosis, or programmed cell death. This mechanism of action effectively targets the cancer cells’ dependence on the abnormal proteins for their survival.
Biosimilars and Ixazomib Citrate API
The arrival of biosimilars for Ixazomib Citrate API disrupts the status quo in MM treatment. While Ixazomib Citrate API has proven effective, its high cost can limit patient access. Biosimilars offer a potential solution by providing near-identical efficacy at a lower price point. This can translate to significant benefits:
- Increased Affordability: Biosimilars can significantly reduce treatment costs for MM patients, improving access to this critical therapy. This can be particularly beneficial for patients with limited insurance coverage or high out-of- pocket expenses.
- Enhanced Healthcare System Sustainability: Lower treatment costs associated with biosimilars can lessen the financial burden on healthcare systems, allowing for better resource allocation across different treatment options.
- Potential for Innovation: The presence of biosimilars can stimulate innovation from the manufacturers of Ixazomib Citrate API. This could lead to developments like improved delivery methods, combination therapies, or even new formulations of Ixazomib Citrate API itself.
However, the biosimilar landscape for Ixazomib Citrate API is still evolving, and some uncertainties remain:
- Long-Term Safety and Efficacy Data: While biosimilars undergo rigorous testing, long-term data on their safety and efficacy compared to the original biologic (Ixazomib Citrate API) is still being collected. This may raise some initial concerns among patients and healthcare providers.
- Switching Considerations: Transitioning patients from Ixazomib Citrate API to a biosimilar may require careful monitoring and potential adjustments to treatment regimens. Healthcare providers will need to weigh the benefits of cost savings with the potential need for close monitoring during the switch.
- Physician and Patient Choice: Ultimately, the decision to use Ixazomib Citrate API or a biosimilar will lie with physicians and patients, considering factors like individual patient characteristics, treatment history, and cost considerations
The Future of Ixazomib Citrate API in the Biosimilars Age
The future of Ixazomib Citrate API in the age of biosimilars looks promising, offering several significant benefits. One key advantage is broader clinical use due to reduced costs, making advanced treatments more accessible to a wider patient population. This increased accessibility can improve outcomes for those with conditions such as multiple myeloma.
Biosimilars also drive further research and development, encouraging innovation that can optimize the efficacy and safety of Ixazomib Citrate API. Researchers might develop new therapeutic regimens and delivery methods, enhancing the overall effectiveness of the treatment.
Additionally, biosimilars have a global impact by facilitating the distribution of advanced therapies worldwide. Patients in developing countries, who might have limited access to expensive biologics, could benefit from effective treatments involving Ixazomib Citrate API, addressing healthcare disparities and improving outcomes on a global scale.
Cost savings are another critical aspect, as lower drug costs can alleviate the financial burden on healthcare systems and patients. These savings can be redirected to other healthcare areas, enhancing the system’s overall efficiency and effectiveness.
Conclusion
Ixazomib Citrate API has established itself as a valuable treatment for MM. The arrival of biosimilars presents both challenges and opportunities. By adapting to the changing market landscape and potentially lowering costs, Ixazomib Citrate API can continue to be a relevant treatment option for MM patients in the age of biosimilars.