Ixazomib Citrate API: A Weapon for Multiple Myeloma Treatment
Multiple myeloma is a cancer of plasma cells, a type of white blood cell found in bone marrow. These plasma cells normally produce antibodies to help fight infections. In multiple myeloma, however, abnormal plasma cells multiply uncontrollably and produce abnormal proteins that can damage bones, kidneys, and other organs. Treatment options for multiple myeloma have evolved significantly in recent years, with the introduction of novel therapies like Ixazomib Citrate API.
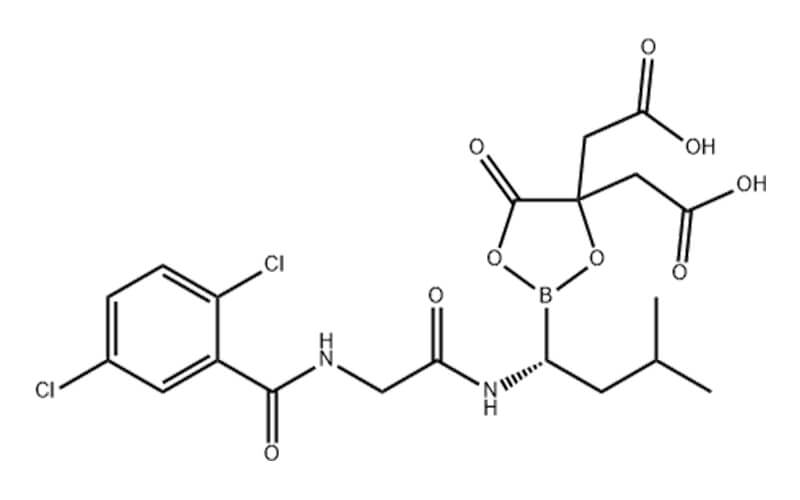
What is Ixazomib Citrate API?
Ixazomib Citrate API is an active pharmaceutical ingredient (API). An API is the pure, essential component of a medication that produces the desired therapeutic effect. Ixazomib Citrate API itself is not directly administered to patients. Instead, it serves as the building block for the final drug product, Ninlaro, which is a prescription medication used to treat multiple myeloma.
The chemical formula of Ixazomib Citrate is C20H23BCl2N2O9, and its molecular weight is approximately 517.12 grams per mole. Once formulated into the final drug product, Ninlaro is typically administered as capsules taken orally.

Mechanism of Action of Ixazomib Citrate
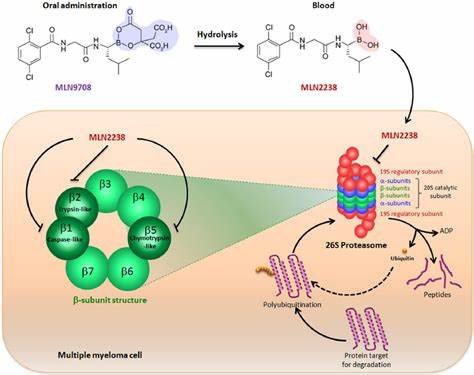
The mechanism of action of ixazomib citrate is centered on its ability to inhibit the proteasome. The proteasome is responsible for degrading a large proportion of intracellular proteins, including those involved in regulating the cell cycle, signaling pathways, and apoptosis (programmed cell death). By inhibiting the proteasome, ixazomib citrate disrupts these vital processes, leading to an accumulation of proteins within the cell. This accumulation induces stress within the cell, eventually triggering apoptosis, particularly in rapidly dividing cells such as cancer cells.
In the context of multiple myeloma, ixazomib citrate interferes with the growth and survival of myeloma cells. Multiple myeloma is characterized by the proliferation of malignant plasma cells in the bone marrow. These cells rely heavily on the proteasome for survival due to their high rate of protein synthesis and turnover. By inhibiting the proteasome, ixazomib citrate induces apoptosis in these malignant cells, reducing tumor burden and slowing disease progression.
Clinical Applications and Efficacy of Ixazomib Citrate
The primary clinical application of Ixazomib Citrate is in the treatment of multiple myeloma. It is typically used in combination with other medications, such as lenalidomide and dexamethasone, as part of a treatment regimen. Studies have shown that Ixazomib Citrate, in combination with other therapies, can improve progression-free survival (PFS) in patients with multiple myeloma who have received at least one prior treatment. PFS refers to the length of time a patient lives without their cancer worsening.
Here’s a breakdown of Ixazomib Citrate’s efficacy compared to other treatments:
- Comparison to Bortezomib: Bortezomib is another proteasome inhibitor used to treat multiple myeloma. Studies have shown that Ixazomib Citrate, in combination with lenalidomide and dexamethasone, may be associated with a longer PFS compared to Bortezomib used with the same combination.
- Comparison to Standard Therapy: For patients who have received at least one prior treatment, Ixazomib Citrate combined with lenalidomide and dexamethasone has been shown to be more effective than treatment with lenalidomide and dexamethasone alone.
Side Effects of Ixazomib Citrate
As with any medication, Ixazomib Citrate can cause side effects. It is important to note that these side effects are associated with the formulated drug Ninlaro, not the isolated Ixazomib Citrate API.
The most common side effects reported with Ixazomib Citrate include:
- Diarrhea: This is a frequent side effect, and can sometimes be severe. Doctors may prescribe medications to help manage diarrhea.
- Fatigue: Many patients experience tiredness during treatment with Ixazomib Citrate. Getting enough rest and maintaining a balanced diet can help alleviate fatigue.
- Peripheral neuropathy: This refers to nerve damage in the hands and feet, which can cause numbness, tingling, or pain. Doctors may recommend medications or physical therapy to manage neuropathy.
- Thrombocytopenia: This is a decrease in the number of platelets, a type of blood cell involved in clotting. Thrombocytopenia can increase the risk of bleeding. Regular blood tests are necessary to monitor platelet levels.
- Thrombocytosis: Ixazomib Citrate can also lead to an increase in platelet count. While generally not a serious issue, it requires monitoring by a doctor.
- Respiratory infections: Ixazomib Citrate can weaken the immune system, making patients more susceptible to infections. Frequent handwashing and avoiding contact with sick people can help minimize this risk.
Other side effects may also occur. It is crucial for patients to discuss all potential side effects with their doctor before starting treatment with Ixazomib Citrate.
Precautions for Using Ixazomib Citrate in the Treatment of Multiple Myeloma
There are several precautions to consider when using Ixazomib Citrate (formulated as Ninlaro) in the treatment of multiple myeloma:
- Pregnancy and breastfeeding: Ixazomib Citrate can harm an unborn baby. Women who are pregnant or planning to become pregnant should not take Ninlaro. Effective contraception is essential during treatment. Ixazomib Citrate can also pass into breast milk, potentially harming a nursing infant. Breastfeeding is not recommended while taking Ninlaro.
- Liver problems: Ixazomib Citrate can affect the liver. Patients with existing liver problems may need their dosage adjusted or may not be suitable candidates for treatment.
- Kidney problems: Ixazomib Citrate is eliminated from the body primarily through the kidneys. Patients with severe kidney dysfunction may experience increased side effects. Dosage adjustments or alternative treatments may be necessary.
- Peripheral neuropathy: As mentioned earlier, Ixazomib Citrate can cause nerve damage. Patients with pre-existing neuropathy may experience worsening symptoms. Doctors will closely monitor patients for signs of neuropathy and may adjust the dose or discontinue treatment if necessary.
- Blood cell counts: Ixazomib Citrate can affect blood cell counts, including a decrease in platelets (thrombocytopenia) or an increase in platelets (thrombocytosis). Regular blood tests are necessary to monitor these counts and manage any potential bleeding risks.
- Drug interactions: Ixazomib Citrate can interact with other medications. It is crucial to inform your doctor about all medications, supplements, and herbal remedies you are taking before starting treatment with Ixazomib Citrate.
- Vaccinations: Live vaccines should be avoided while taking Ixazomib Citrate due to a weakened immune system. Discuss vaccination needs with your doctor before starting treatment.
It is important to remember that this is not an exhaustive list of precautions. Patients considering Ixazomib Citrate therapy should discuss all potential risks and benefits with their doctor in detail. Open communication and close monitoring throughout treatment is essential for ensuring patient safety and maximizing the effectiveness of Ixazomib Citrate.
Conclusion
Ixazomib Citrate API represents a significant advancement in the fight against multiple myeloma. By inhibiting proteasomes, it disrupts a critical pathway for cancer cell survival and growth. Clinical trials have shown promising results for Ixazomib Citrate in combination with other therapies for multiple myeloma. However, like any medication, Ixazomib Citrate can cause side effects, and there are important precautions to consider before starting treatment. Open communication with a healthcare professional is essential to determine if Ixazomib Citrate is the right treatment option for an individual with multiple myeloma.
If you are seeking a reliable and high-quality source of Ixazomib Citrate API, Qingmu stands out as a trusted manufacturer. Qingmu is renowned for its commitment to excellence and stringent quality control processes, ensuring that its Ixazomib Citrate API meets the highest industry standards. Please feel free to contact us for your Ixazomib Citrate API needs, you’ll get professional and kind service.