Brivaracetam API: A Key Component in Epilepsy Treatment
Brivaracetam API is a pharmaceutical intermediate used in the production of the medication Briviact, which is prescribed to treat partial-onset seizures. This article will delve into the key aspects of Brivaracetam API, including its definition, applications, mechanism of action (as much as is currently known), drug class, and prominent manufacturers, with a particular focus on Qingmu pharmaceutical.
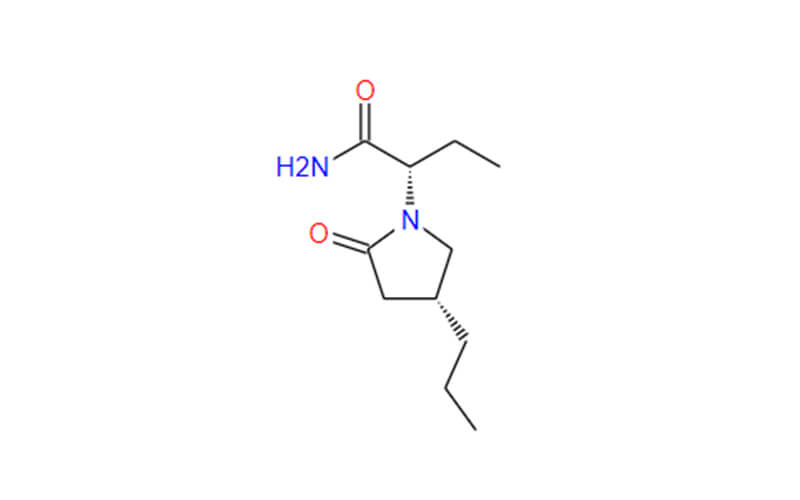
What is Brivaracetam API?
Brivaracetam API, short for Brivaracetam Active Pharmaceutical Ingredient, is a chemically synthesized compound that serves as the primary active component in the prescription drug Briviact. Brivaracetam itself belongs to the class of compounds known as racetams, which are characterized by a specific chemical structure and have been shown to exhibit anticonvulsant properties.
While the precise mechanism by which Brivaracetam API exerts its anticonvulsant effects is still under investigation, research suggests that it may be linked to its interaction with specific proteins and ion channels within the nervous system.
Applications of Brivaracetam API
The primary application of Brivaracetam API lies in the treatment of partial-onset seizures, a type of seizure that originates in a localized area of the brain. These seizures can manifest in various ways depending on the specific location of the originating activity within the brain. Some individuals may experience motor symptoms such as uncontrolled muscle movements or sensations of tingling or numbness. Others may have alterations in perception, hearing, or smell. In some cases, partial-onset seizures may progress to generalized seizures, which involve abnormal electrical discharges that spread throughout the entire brain.
Brivaracetam API is typically administered as an adjunctive therapy, meaning it is used alongside other anticonvulsant medications to improve seizure control in patients who have not achieved satisfactory outcomes with single-drug therapy.
Mechanism of Action of Brivaracetam API
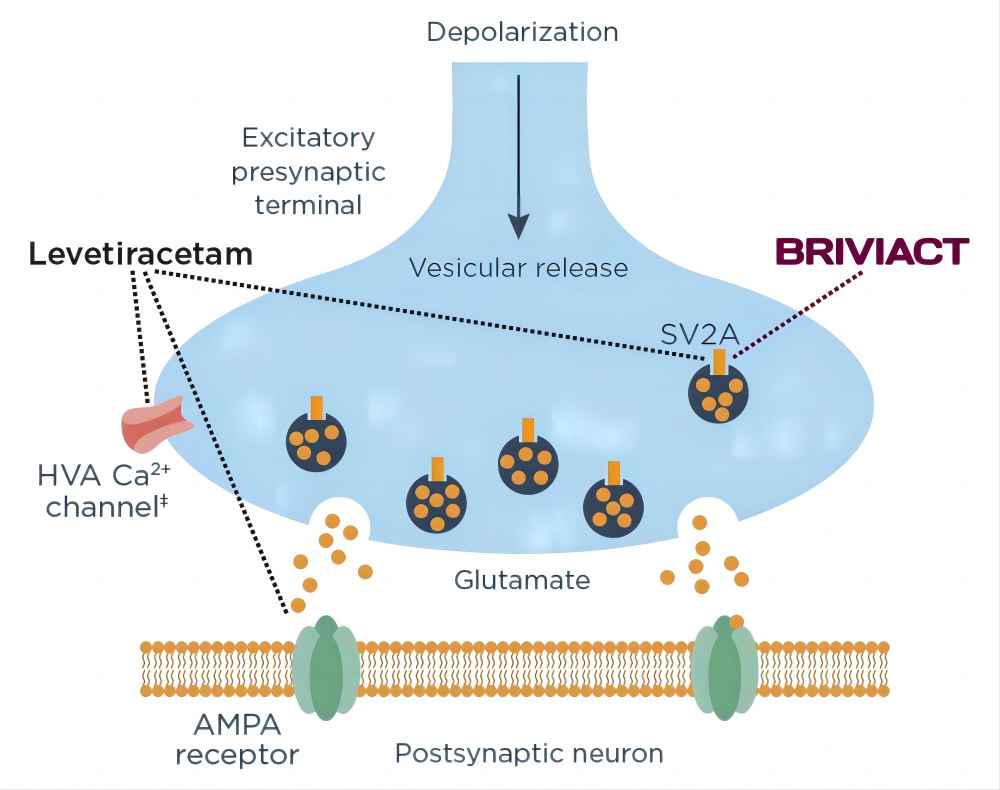
The exact mechanism by which Brivaracetam API exerts its anticonvulsant effects remains under scientific exploration. However, current research suggests that it may be involved in modulating the activity of certain neurotransmitters and ion channels in the brain.
One potential mechanism involves the interaction of Brivaracetam API with a protein called synaptic vesicle protein 2A (SV2A). SV2A is found in synaptic vesicles, which are tiny sacs within nerve cells that store neurotransmitters. When nerve cells communicate with each other, they release neurotransmitters from these synaptic vesicles. Some researchers believe that Brivaracetam API may bind to SV2A, potentially influencing the release of neurotransmitters and thereby regulating neuronal excitability.
Another proposed mechanism of action centers on the modulation of sodium channels. Sodium channels are pore-like proteins embedded in the membranes of nerve cells that play a crucial role in the transmission of nerve impulses. By regulating the activity of sodium channels, Brivaracetam API may help stabilize neuronal activity and prevent the excessive firing of neurons that can contribute to seizures.
It is important to note that these are just two of the possible mechanisms by which Brivaracetam API may work. Further research is needed to fully elucidate the precise way in which it exerts its anticonvulsant effects.
Drug Class of Brivaracetam API
Brivaracetam API belongs to the class of anticonvulsant medications. Anticonvulsant medications are a diverse group of drugs that are used to prevent or control seizures. They work by various mechanisms to stabilize abnormal electrical activity in the brain.
Brivaracetam API is further classified as a racetam. Racetams are a subgroup of anticonvulsant medications that share a common chemical structure. Other well-known racetams include piracetam, levetiracetam, and oxcarbazepine. While all racetams exhibit anticonvulsant properties, the specific mechanisms by which they work can differ.
Briviact Manufacturer: Qingmu Pharmaceutical and Its Qualifications
Briviact, the brand-name medication containing Brivaracetam API, is manufactured by several pharmaceutical companies around the world. One prominent manufacturer is Qingmu, a Chinese pharmaceutical company with a well-established reputation for producing high-quality Active Pharmaceutical Ingredients (APIs).
Qingmu adheres to strict quality control standards throughout the manufacturing process of Brivaracetam API. This ensures that the final product meets the necessary purity, potency, and safety specifications. The company employs advanced manufacturing technologies and well-trained personnel to guarantee the consistent quality of its Brivaracetam API.
In addition to its commitment to quality, Qingmu is also known for its reliable supply chain and competitive pricing. This makes them a trusted partner for pharmaceutical companies that develop and manufacture Briviact and other medications containing Brivaracetam API.
Considerations for Using Brivaracetam API
While Brivaracetam API offers a promising approach to treating partial-onset seizures, there are some important considerations to keep in mind:
- Dosage and Administration: Brivaracetam API is typically formulated into tablets or capsules for oral administration. The specific dosage regimen for a patient will be determined by a healthcare professional based on various factors such as the severity of their seizures, age, and overall health condition.
- Safety and Side Effects: Like all medications, Brivaracetam API can cause side effects in some individuals. Common side effects include dizziness, drowsiness, fatigue, headache, and nausea. In rare cases, more serious side effects can occur. It is crucial for patients taking Brivaracetam API to be aware of the potential side effects and to promptly report any concerning symptoms to their healthcare provider.
- Drug Interactions: Brivaracetam API can interact with other medications. Patients taking Brivaracetam API should inform their healthcare professional about all other medications, both prescription and over-the-counter, that they are currently taking. This will help to minimize the risk of potential drug interactions.
- Research and Development: Research into Brivaracetam API and its mechanism of action is ongoing. Scientists are continually seeking to gain a deeper understanding of how it works and how it can be further optimized for the treatment of epilepsy. Additionally, researchers are exploring the potential applications of Brivaracetam API for other neurological conditions beyond epilepsy.
Conclusion
Brivaracetam API is a valuable tool in the treatment of partial-onset seizures. While the exact way it works is still being investigated, its potential to modulate neurotransmitter release and sodium channel activity offers a promising approach for controlling abnormal electrical activity in the brain. As research continues, Brivaracetam API may play an increasingly important role in the management of epilepsy and potentially other neurological disorders.