A Deep Look into Mirogabalin API: Market, Synthesis, and Application
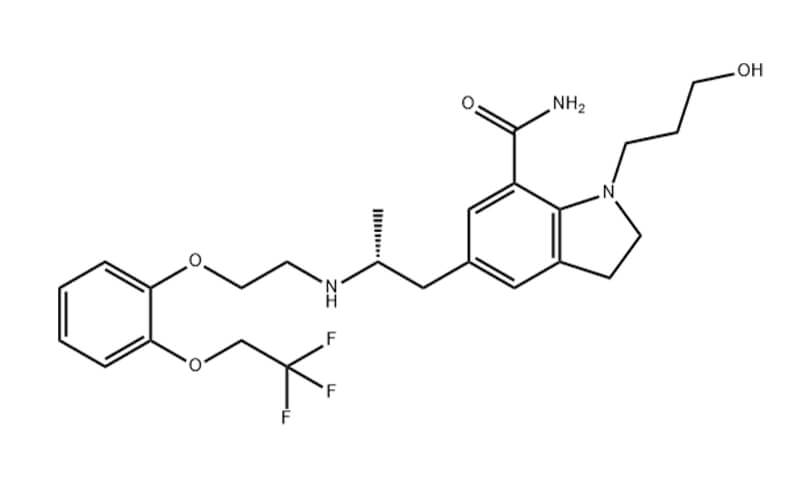
Mirogabalin API is a pharmaceutical API with a CAS number of 1138245-13-2, a molecular formula of C12H19NO2, and a molecular weight of 209.28. Mirogabalin API is commonly used to treat pain associated with diabetic peripheral neuropathy, fibromyalgia, and postherpetic neuralgia.
What is the Market Situation of Mirogabalin API?

Mirogabalin active pharmaceutical ingredient(Mirogabalin API), as an important pharmaceutical intermediate, continues to grow in demand in the global market. According to the latest market data, the global market size of active pharmaceutical ingredients reached $204.04 billion in 2022, and is expected to reach $363.7 billion by 2032, showing a compound annual growth rate of 6%. This strong growth momentum reflects the overall prosperity of the global pharmaceutical market.
The competitive landscape of Mirogabalin API in the global market presents diversified and regional characteristics. The global raw material pharmaceutical industry is undergoing a process of reshaping, especially in the wave of globalization and marketization, where governments around the world are increasingly valuing the development of the raw material pharmaceutical industry.
On a global scale, the competitive landscape of the active pharmaceutical industry presents an oligopoly trend, with high industry concentration and a focus on original research. For example, in the contrast agent raw material market, companies such as GE, Guerbet, Bracco, Bayer, etc. occupy the majority of the market share.
For Mirogabalin API, their competitive landscape in the global market may be similar to other active pharmaceutical ingredients, influenced by various factors, including but not limited to market demand, production costs, technological innovation, policy environment, etc.
It is worth noting that China and India are the main sources of global supply of active pharmaceutical ingredients, and both countries have significant advantages in the production and export of active pharmaceutical ingredients. China’s production capacity of raw materials accounts for about 28% of the world’s total, with approximately 65% of the produced raw materials used for export, mainly for bulk raw materials. India is also an important producer and exporter of raw materials, but its raw material production capacity has been greatly affected during the pandemic.
As the demand for Mirogabalin raw materials continues to grow, the global market is expected to further expand and diversify. New participants may enter the market, which may lead to intensified competition and innovation. Understanding these trends is crucial for buyers seeking reliable and cost-effective sources of Mirogabalin APIs.
What are the Common Synthesis Routes for Mirogabalin API?
Mirogabalin is a new gabapentin drug that contains a hydrophobic bicyclic substituent in its chemical structure and can specifically bind to the voltage-gated calcium channel subunit α2δ1. This medication is primarily used to treat peripheral neuropathic pain (PNP), such as diabetic peripheral neuropathic pain (DPNP) and postherpetic neuralgia (PHN)
It can be synthesized through various routes. Here’s a look at two common methods employed for its production:
1. Asymmetric Synthesis:
This method leverages chiral catalysts to achieve a highly specific outcome – the production of enantiomerically pure Mirogabalin API. Enantiomers are mirror image molecules with identical chemical formulas but slightly different spatial arrangements. In pharmaceuticals, achieving enantiomeric purity is crucial as only one enantiomer often possesses the desired therapeutic effect.
Asymmetric synthesis utilizes chiral catalysts, which act like microscopic matchmakers, preferentially binding to one specific enantiomer of a starting material during the reaction. This preferential binding steers the reaction towards the formation of the desired enantiomer of Mirogabalin API, ensuring a potent and effective final product.
2. Resolution Process:
This approach takes a different route. Here, a racemic mixture of Mirogabalin API is first produced, containing both the desired and unwanted enantiomers. Subsequently, the resolution process separates the desired enantiomer from the unwanted one. Various techniques can be employed for resolution, such as:
- Diastereomeric Salt Formation: In this method, the racemic mixture is reacted with a chiral resolving agent to form diastereomers – distinct molecular structures that can be separated due to their differing physical properties like solubility. Once separated, the desired enantiomer of Mirogabalin API can be retrieved from its corresponding diastereomer.
- Chromatographic Separation: This technique utilizes a stationary phase (a solid material) and a mobile phase (a liquid or gas) to separate the enantiomers based on their differing interactions with these phases. The desired enantiomer of Mirogabalin API will have a slightly different affinity for the stationary phase compared to the unwanted enantiomer, allowing for their separation.
The choice between asymmetric synthesis and the resolution process depends on various factors, including cost-effectiveness, reaction efficiency, and the availability of suitable chiral catalysts or resolving agents.
How is Mirogabalin API Commonly Used in Skin Care Products?
Mirogabalin API, while still under research for its applications in skincare, shows promise for its potential to address various skin concerns. Here’s a breakdown of how it’s being explored for use in skincare products:
1. Soothing Itch:
One of the most exciting potential applications of Mirogabalin API lies in its ability to alleviate itching. Itching is a common symptom associated with numerous skin conditions, including:
- Eczema: This inflammatory skin condition causes intense itching along with dry, cracked skin and redness. Mirogabalin API’s potential to modulate nerve signaling suggests it might bring relief from eczema-related itching.
- Psoriasis: Psoriasis is an autoimmune condition characterized by red, itchy patches of thickened skin. Mirogabalin API’s anti-itching properties could potentially provide comfort for psoriasis sufferers.
- Insect Bites: Itchy insect bites can be a real nuisance. Topical formulations containing Mirogabalin API might offer soothing relief by interrupting the itch signal at the nerve level.
2. Reducing Inflammation:
Studies suggest Mirogabalin API might possess anti-inflammatory properties, making it potentially beneficial for conditions marked by inflammation:
- Acne: Acne is an inflammatory skin condition characterized by pimples, blackheads, and cysts. Mirogabalin API’s anti-inflammatory effects could potentially help reduce inflammation and promote healing in acne lesions.
- Rosacea: Rosacea causes facial redness, inflammation, and visible blood vessels. Mirogabalin API, if proven effective, could potentially reduce inflammation and improve the appearance of rosacea symptoms.
3. Pain Relief:
Mirogabalin API’s pain-relieving properties could offer benefits for skin conditions associated with discomfort:
- Sunburn: Sunburn causes inflammation, pain, and redness. Topical formulations containing Mirogabalin API might offer soothing relief by potentially reducing pain signals.
- Post-procedure Care: Skin procedures like laser treatments or chemical peels can cause temporary discomfort and redness. Mirogabalin API could potentially reduce pain and promote healing after these procedures.
Important Considerations:
It’s crucial to remember that the use of Mirogabalin API in skincare products is still under investigation. While preliminary research is promising, more extensive clinical trials are needed to definitively establish its efficacy and safety for various skin conditions. Always consult with a qualified dermatologist or healthcare professional before using any skincare product containing Mirogabalin API.
Mirogabalin API Manufacturer: Qingmu Pharmaceutical

Qingmu Pharmaceutical stands at the forefront of Mirogabalin API production, renowned for its commitment to quality, innovation, and customer satisfaction. With state-of-the-art manufacturing facilities and a dedicated research and development team, we deliver premium-grade Mirogabalin API that meets the stringent requirements of pharmaceutical and cosmetic industries worldwide. By leveraging advanced synthesis technologies and adhering to strict regulatory standards, Qingmu Pharmaceutical ensures the reliability, purity, and consistency of its Mirogabalin API offerings, positioning itself as a trusted partner for discerning buyers seeking high-quality pharmaceutical ingredients.
If you are interested in Qingmu or our Products, please feel free to contact us.